Spray drying as an approach for enhancement of dissolution and bioavailability of Raloxifene hydrochloride.
Keywords:
Dissolution enhancement, Bioavailability, Spray drying, roloxifene, poorly soluble drugAbstract
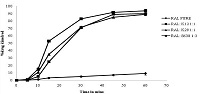
The present study investigated the effect of spray drying raloxifene HCl (RHCL) with different classes of hydrophilic carriers (different grades of polyvinyl pyrrolidones) and cellulosic polymers) in order to determine the potential effect on dissolution rate and bioavailability of RHCL. Preformulation studies were conducted to select the appropriate carriers and drug:carrier ratio for preparing the spray dried compositions.The solid state interactions of the spray dried mixtures were evaluated by DSC & XRD. Preformulation studies revealed that amorphous compositions of RHCL could be obtained only with Plasdones (K12, K29/32 and S630). DSC studies showed that the crystalline nature of RHCL was significantly reduced on spray drying. Significant enhancement in dissolution rate was observed with the prepared spray dried compositions and out of the three grades of Plasdone, Plasdone K12 demonstrated the maximum enhancement in rate of release of RHCL. The pharmacokinetics of spray dried composition (1:1 RHCL: K12) and pure RHCL was evaluated following oral administration (25 mg/kg) in healthy female Sprague Dawley rats. The extent of the mean plasma exposures of RHCL was 7-fold higher in animals treated with spray dried mixture of RHCL, K12 (1:1) compared to animals treated with RHCL. Spray drying of RHCL with Plasdones, especially Plasdone K12, reduced drug crystallinity, increased the rate and extent of dissolution, and improved bioavailability..
References
Kerry JH, Gordon NA, Steven WB, Ross AJ, Wayne DL, Neil GP, Eugene CR, Cheryl AT, Paul KST, Robert EW. Influence of peroxide impurities in povidone and crospovidone on the stability of raloxifene hydrochloride in tablets: identification and control of an oxidative degradation product. Pharm Dev Tech. 2000; 5(3): 303-308.
Delmas PD, Bjarnason N H, Mitlak B, Ravoux AC, Shah A, Huster W, Draper M, Christiansen C. Effects of Raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N.Engl. J. Med 1997; 337: 1641–1647.
Mitlak BH, Cohen FJ. In search of optimal long term female hormone replacement: the potential of selective estrogen receptor modulators.Horm Res 1997; 48:155–163.
Garg A, Singh S, Rao VU, Bindu K, Balasubramaniam J. Solid State Interaction of Raloxifene HCl with Different Hydrophilic Carriers during Co-grinding and its Effect on Dissolution Rate. Drug Dev. Ind. Pharm 2009; 35(4): 455-470.
Martin A. 2003 Physical Pharmacy fourth ed. Williams and Wilkins Baltimore. p.231-240.
Patterson JE, James MB, Forster AH, Lancaster RW, Butler JM., Rades T. Preparation of glass solutions of three poorly water soluble drugs by spray drying, melt extrusion and ball milling. Int J. Pharm. 2007; 336: 22-34.
Chaumeil JC. A method of improving the bioavailability of poorly soluble drugs. Methods Find. Exp. Clin. Pharmacol .(1998) 20: 211–215
Otsuka M, Kaneniwa N. Effects of grinding on the physicochemical properties of cephalexin. Chem. Pharm. Bull. 1984; 32: 1071–1079.
Friedrich H, Nada A, Bodmeier R. Solid state and dissolution rate characterization of Co-ground mixtures of nifedipine and hydrophilic carriers. Drug Dev.Ind.Pharm. 2005; 31: 719-728.
Sugimoto M, Okagaki T, Narisawa S, Koide Y, Nakajima K. Improvement of dissolution characteristics and bioavailability of poorly water-soluble drugs by novel co-grinding method using water-soluble polymer. Int. J. Pharm 1998; 160: 11–19.
Suzuki H, Ogawa M, Hironaka K, Ito K , Sunada H. A nifidipine co-grpund mixture with sodium deoxycholate. I. Colloidal particle formation and solid state analysis. Drug Dev Ind Pharm. 2001; 27 (9): 943-949.
Ahuja N, Katare OP, Singh B. Studies on dissolution enhancement and mathematical modeling of drug release of poorly water-soluble drug using water soluble carriers. Eur. J Phar. Biopharm. 2007; 65: 26-38.
Masters K 2002. Spray drying in practice. In: SprayDry Consult International ApS (Eds.), Charlo ttenlund, Denmark.
Giunchedi P, Conte U. Spray drying as a preparation method of microparticulate drug delivery systems: An overview. S. T. P. Pharm. Sci . 1995; 5 : 276-290.
Giunchedi P, Conti B, Genta I, Conte U, Puglisi G. Emulsion spray drying for the preparation of albumin loaded PLGA microspheres. Drug Dev. Ind. Pharm. 2001; 27: 745-750.
Alanzi FK, El-Badry M, Ahmed MO, Alsarra IA. Spray-dried HPMC microparticles of indomethacin: impact of drug-polymer ratio and viscosity of the polymeric solution on dissolution. Sci. Pharm. 2007; 75: 63-79.
Chen R, Tagawa M, Hoshi N, Ogura T, Okamoto H, Danjo K. Improved dissolution of an insoluble drug using a 4-fluid nozzle spray drying technique. Chem. Pharm. Bull 2004; 52: 1066-1070.
Filipovic-Grcic J, Perissutti B, Moneghini M, Voinovich D, Martinac A, Jalsenjak I. Spray dried carbamazepine loaded chitosan and HPMC microspheres: preparation and characterization. J. Pharm. Pharmacol. 2003; 55: 921-931.
Moretti MD, Gavini E, Juliano C, Pirisino G, Giunchedi P. Spray dried microspheres containing ketoprofen formulated into capsules and tablets. J. Microencapsui. 2001;18:111-121.
Alanzi FK, El-Badry M, Alsarra IA. Improvement of Albendazole dissolution by preparing microparticles using spray-drying technique. Saudi Pharm. J. 2006; 14: 100-107.
Balasubramaniam J, Bindu K, Rao VU, Ray D, Haldar R, Brzeczko AW. Effect of superdisintegrants on dissolution of cationic drugs. Dissol. Technol. 2008; May: 18-25.
Hancock BC, Zografi G. Characteristic and significance of the amorphous state in pharmaceutical systems. J. Pharm. Sci. 1997; 86: 1–12.
Williams AC, Timmins P, Lu M, forbes RT. Disorder and dissolution enhancement: Deposition of ibuprofen on to insoluble polymers. Eur. J. Pharm. Sci. 2005; 26: 288-294.
Betageri GV, Makarla KR. Enhancement of dissolution of glyburide by solid dispersion and lyophilization techniques. Int. J. Pharm 1995; 126: 155–160.
Valizadeh H, Nokhodchi A, Qarakhani N, Zakeri-Milani P, Azarmi S, Hassanzadeh D, Lobenberg R. Physicochemical characterization of solid dispersions of indomethacin with PEG 6000, Myrj 52, lactose, sorbitol, dextrin, and Eudragit® E100. Drug Dev. Ind. Pharm 2004; 30: 303–317.
Vippangunta SR, Maul KA, Tallavajhala S, Grant DJW. Solid state characterization of nifedipine solid dispersion. Int. J. Pharm. 2002; 236: 111-126.
Hancock BC, Parks M. What is true solubility advantage for amorphous pharmaceuticals? Pharm Res. 2000; 17 (4): 397-403.