Formulation And Evaluation Of Anti-Ulcer Floating Tablet Using Swellable Polymers
Keywords:
Floating drug delivery, Natural polymers, Synthetic polymers, Ranitidine HydrochlorideAbstract
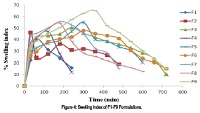
Present study involves the formulation and evaluation of floating tablets Ranitidine hydrochloride by direct compression method by using HPMC K4M, HPMC K100M as a synthetic polymers and Gellan Gum (low acyl) as a natural polymer with addition of sodium bicarbonate and citric acid as effervescent agent. The physicochemical compatibility of the drug and the polymers was studied by infrared spectroscopy and differential scanning calorimetry. The results suggested that drug and the polymers were physicochemically compatible with each other. The effect of synthetic and natural polymers on the drug release and floating properties of tablet were investigated. Formulation was optimized on the basis of pre compression and post compression parameters, floating lag time, total floating time and in vitro drug release study was carried out. The floating lag time, dissolution studies indicated that formulation F11 with drug: polymer ratio 5:4 exhibited sustained release of drug and followed Korsemeyer Peppas kinetics. Natural polymer (Gellan Gum) showed better results for sustained drug release properties than synthetic polymer. The floating lag time was found to be increase significantly with increase in concentration of polymer and drug release was found to decrease with increase concentration of polymers
References
. Shaji J, Patole V. Protein and peptide drug delivery: Oral approaches. Indian J Pharm Sci 2008;70:269-277
. Nayak A, Maji R and Das B. Gastroretentive drug delivery system; Review. Asian. J. Pharm. Res 2010; 3, 2-10.
. Mishra A, Gupta P. Gastro Retentive Drug Delivery System: A Review. Int. J. Drug Dev. & Res 2012; 4 (4): 28-39.
. Chawla, G., Gupta, P., Koradia, V. and Bansal, A. K., Gastroretention- A means to address regional variability in intestinal drug absorption. Pharm.Tech 2003; 27(7): 50-51.
. Shah SH, Patel JK, Patel NV. Stomach specific floating drug delivery system: A review. Int J Pharm Tech Res 2009; 1:623-633.
. Wilson CG, Washington N. Physiological Pharmaceutics: Biological Barriers to Drug Absorption, Horwood Ellis, Chichester 1989;47-70.
. Brijesh SD, Avani FA, Madhabhai MP, Gastroretentive Drug Delivery System of Ranitidine Hydrochloride Formulation and Invitro Evaluation; Aaps Pharm Sci Tech 2004;5(2):1-6.
. Abdul WB, Larry FL. Colonic Metabolism of Ranitidine, Implications For its Delivery and Absorption; Int J Pharm 2001;227:157-165.
. Description and clinical pharmacology of Zantac® Tablets (Ranitidine Hydrochloride USP) 56th ed. Medical economic company 2002; Physician’s Desk reference.
. Robert MS, Francis XW. Infrared Spectrometry. In: Robert M. Silverstein. Editors. Spectrometric Identification of Organic Compounds. 6th Ed. John Wiley and Sons. Inc. New York: 71-143.
. Indian pharmacopoeia 2007, Ghaziabad, India: Indian Pharmacopoeia Commission 2007; 1: 182-183.
. Marshall K, Lachman N, Liberman HA. The theory and practice of industrial pharmacy, 3rd edition. Mumbai: Varghese publishing house 1987;66-69.
. United States Pharmacopeia and National Formulary USP 27–NF 22; The United States Pharmacopeial Convention, Inc.:Rockville, MD 2004;454–458.
. Ghosh NS, Ghosh S, Debnath S. Formulation Of Immediate Dosage Form Of Ranitidine Hydrochloride Tablets Using HPMC And Starch Acetate Film Former; J Chem Pharm Res 2010;2:147-157.
. Xu Xiaoqiang, Sun Minjie, Zhi Feng, Hu Yiqiao. Floating matrix dosage form for phenoporlamine hydrochloride based on gas forming agent: In vitro and in vivo evaluation in healthy volunteers; International Journal of Pharmaceutics 2006;310:139–145.
. Verma S, Narang N. Development And In Vitro Evaluation Of Floating Matrix Tablets Of Anti Retroviral Drug; Int J Pharm Sci 2011;3:208-211.
. Cartensen JT. Drug Stability: Principle and practice. Second edition, Marcel Dekker, New York 1995;538-550.
. Chen YH, Yaung JF. Alka Seltzer fizzing- determination of percent by mass of sodium bicarbonate in alka seltzer tablets. J Chem Ed 2002;79:848-850.
. Patil VS, Gaikwad PD, Bankar VH and Pawar SP. Formulation And Evaluation of Floating Matrix Tablet of Locally Acting H2-Antagonist; Int J of Pharm and Tech 2010;2:528-540.
. Enayatifard R, Saeedi M, Akbari J, Tabatabaee YH. Effect of hydroxypropyl methylcellulose and ethyl cellulose content on release profile and kinetics of diltiazem HCl from matrices. Trop J Pharm Res 2009;8:425-432.
. Dhawan S, Varma M, Sinha VR. High molecular weight poly(ethylene oxide) – based drug delivery systems Part I: Hydrogels and hydrophilic matrix systems. Pharm Technol 2005;29:72-79.