Formulation and evaluation of poly (L-lactide-co-ē-caprolactone) loaded gliclazide biodegradable nanoparticles as a control release carrier
Keywords:
Poly (L-lactide-co-ē-caprolactone), Gliclazide, Biodegradable Nanoparticles, Lyophilization, High Pressure HomogenizationAbstract
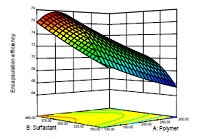
A biodegradable nanoparticle has been used frequently as drug delivery carrier due to its better encapsulation capacity, sustained/ control release property and less toxicity. Gliclazide (GLZ) is a second generation of hypoglycemic sulfonylurea and acts selectively on pancreatic ß cell to control diabetes mellitus. The objective of this study was to produce controlled release nanoparticles of Gliclazide using poly (L-lactide-co-ē-caprolactone) (PLCL). The method was optimized using design of experiments by employing a 3-factor, 3-level Design Expert (version 8.0.7.1) Statistical Design Software and was subjected to various characterization studies including Field Emission Scanning Electron Microscopy (FE-SEM), X-ray diffraction (XRD), Encapsulation efficiency (%EE), Particle Size Distribution (PSD), etc. Formulated nanoparticles were also subjected to Fourier Transform Infrared Spectroscopy (FT-IR), Differential Scanning Calorimetry (DSC) for studying interaction between drug and polymer and the effect of lyophilization (Freeze Drying) on developed nanoparticles. The release profiles and encapsulation efficiencies are depended on the concentration of PLCL. These data demonstrated the efficacy of the biodegradable polymeric nanoparticles in controlling the gliclazide drug release profile as novel drug delivery system.
References
Arunachalam S, Gunasekaran S. Diabetic research in India and China today: From literature-based mapping to health-care policy. Current Sci. 9 ⁄ 10, 1086–97 (2002).
Nolte MS, Karam JH. Pancreatic hormones and antidiabetic drugs. In: Katzung BG (ed.). Basic and Clinical Pharmacology. Lange Medical Books ⁄ McGraw- Hill Publishing Division, New York. 2001; 711– 34.
Hong, S. S., Lee, S. H., Lee, Y. J., Chung, S. J., Lee, M. H., & Shim, C. K., Accelerated oral absorption of gliclazide in human subjects from a soft gelatin capsule containing a PEG 400 suspension of gliclazide. J. Control. Release, 1998; 51:185–192.
Yue Zhao,Wenna Chen, Qing Cai, Shenguo Wang, Jun Bo, Chi Wu, Erosion Induced Controllable Release of Gliclazide Encapsulated Inside Degradable Polymeric Particles, Macromol. Biosci. 2004; 4:308–313.
Kotwal VB, Saifee M, Inamdar N, Bhise K., Biodegradable polymers: Which, when and who?, Indian J. Pharm. Sci.2007; 69:616-625.
Shammi Goyal, Jitendra Kumar Rai, R. K. Narang, Rajesh K. S., Sulfonyl Ureas For Antidiabetic Therapy, An Overview for Glipizide, Int J Pharmacy Pharm Sci.2010;2(2):1-6.
Pathiraja A.Gunatillake and Raju Adhikari, Biodegradable Synthetic Polymers For Tissue Engineering, European Cells and Materials.2003; 5:1-16.
Khaled Al-Tahami and Jagdish Singh, Smart Polymer Based Delivery Systems for Peptides and Proteins, Recent Patents on Drug Delivery & Formulation.2007;1:65-71.
Patrick B. O’Donnell, James W. McGinity, Preparation of microspheres by the solvent evaporation technique, Advanced Drug Delivery Reviews.1997; 28:25–42.
Ming Li, Olivier Rouaud, Denis Poncelet, Microencapsulation by solvent evaporation: State of the art for process engineering approaches, International Journal of Pharmaceutics. 2008;363:26–39.
Preeti Subhedar, J. B. Naik and D. N. Muley, Effect of Polymer Concentration on Sustained Release Microparticles of Metformin Hydrochloride Prepared by Using Spray Dryer, Polymer-Plastics Technology and Engineering.2010;49: 267–271.
Naik. J. B, Mishra S. et al., Development of sustained release micro/nanoparticles using different emulsification technique-A Review., Int. J. of Pharma& Bioscience.2012;3(4):573-579.
Meltem Cetin, Alptug Atila, Yucel Kadioglu, Formulation and In vitro Characterization of Eudragit L100 and Eudragit L100-PLGA Nanoparticles Containing Diclofenac Sodium, AAPS PharmSciTech.2010; 11(3):1250-1256.
Woo-kyoung Lee, Jong-yeun Park, Eun Hee Yang, Hearan Suh, Sung Hoon Kim, Doo Soo Chung, Kihwan Choi, Chul Woo Yang, Jong-sang Park, Investigation of the factors influencing the release rates of cyclosporin A-loaded micro- and nanoparticles prepared by high-pressure homogenizer, Journal of Controlled Release.2002; 84:115–123.
Naik J B, Mokale V J, Formulation and evaluation of Repaglinide nanoparticles as a sustained release carrier, Novel Science International Journal of Pharmaceutical Science. 2012;1(5):259-266.
Padma V. Devarajan, Ganeshchandra S. Sonavane, Preparation and In Vitro/In Vivo Evaluation of Gliclazide Loaded Eudragit Nanoparticles as a Sustained Release Carriers, Drug Development and Industrial Pharmacy.2007; 33:101-111.
Yuancai Dong, Si-Shen Feng, Poly (D,L-lactide-co-glycolide) (PLGA) nanoparticles prepared by high pressure homogenization for paclitaxel chemotherapy, Int. J. Pharm.2007; 342:208-214.