Dilution Phenomenon in Mixed Surfactant based Self Micro Emulsifying Formulations of Ginger Oleoresin: Ex Vivo and In Vivo Performances
Keywords:
Ginger oleoresin, Dilutable SMEDDS, Mixed surfactant, Bioavailability, Solubilization, Ex vivo permeationAbstract
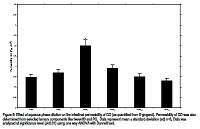
Aqueous solubilization of Ginger Oleoresin (GO) in pseudo self micro emulsifying carrier and its influence on ex-vivo intestinal permeation and in-vivo performances was investigated. GO preconcentrates was prepared using surfactants, Tween 80, Tween 20 and/or 1:1 mixture with a cosurfactant propylene glycol at S/Cos ratio 1:1. Aqueous dilutable region of GO in single or mixed surfactant systems was located from ternary phase diagram drawn between ternary components consisted of surfactant/co-surfactant ratio at 1:1, GO and aqueous phase. Various microstructures were characterized across the dilution line using conductometric and rheological method. Three formulations were selected across the dilution line from mixed surfactants phase diagram as microemulsion area was found to be larger in mixed surfactants over single surfactant based ternary system. GO SMEDDS formulations were physically characterized for refractive index, pH, droplet size and stability assessment. The permeability of GO in diluted pre-concentrate was determined across ex vivo rat intestinal method. Two fold enhancement (p<0.01) in intestinal permeability of GO was obtained from SMEDDS formulation when diluted upto 9.0ml in comparison to under diluted (2 ml) or over-diluted (25 ml) and control formulation (GO in Tween 80). These findings strongly suggested that SMEDDS diluted upto 9ml behave like a pseudo self emulsifying carrier which inherently had microemulsion characteristics (droplet size 122nm). Modulation of intestinal permeability upon dilution was found closely related with dynamics of microemulsion system. Dilution mediated transitions in microstructure of GO SMEDDS was associated with the changes in the orientation of surfactant molecules at the oil-water interface of microstructures during solubilization of GO. In vivo studies revealed that orally administered GO preconcentrate produced 1.6 folds enhancement in oral bioavailability of GO over control. Present study demonstrates that intestinal permeability and oral bioavailability can be modulated via exploration of fully dilutable preconcentrate GO system which could be a possible carrier to enhance oral bioavailability of GO.
References
Grzanna R., Lindmark L, Frondoza CG. Ginger-an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 2005; 8: 125-132.
Zick SM, Turgeon DK, Vareed SK, Ruffin MT, Litzinger AJ, Wright BD, Alrawi S, Normolle DP, Djuric Z, Brenner DE, Phase II study of the effects of ginger root extract on eicosanoids in colon mucosa in people at normal risk for colorectal cancer. Cancer Prev Res (Phila) 2011; 4: 1929-1937.
Kim IS, Kim SY, Yoo HH. Effects of an aqueous-ethanolic extract of ginger on cytochrome P450 enzyme-mediated drug metabolism. Pharmazie. 2012; 67: 1007-1009.
Singh PK, Kaur IP. Development and evaluation of a gastro-retentive delivery system for improved antiulcer activity of ginger extract (Zingiber officinale). J Drug Target 2011; 19: 741-751.
Jiang SZ, Wang NS, Mi SQ. Plasma pharmacokinetics and tissue distribution of [6]-gingerol in rats. Biopharm Drug Dispos 2008; 29: 529-537.
Wei L, Sun P, Nie S, Pan W. Preparation and evaluation of SEDDS and SMEDDS containing carvedilol. Drug Dev Ind Pharm. 2005; 31: 78
Hong JY, et al., A new self-emulsifying formulation of itraconazole with improved dissolution and oral absorption. J Control Rel. 2006. 110: 332-8.
Joshi RP, Negi G, Kumar A, Pawar YB, Munjal B, Bansal AK, Sharma SS. SNEDDS curcumin formulation leads to enhanced protection from pain and functional deficits associated with diabetic neuropathy: An insight into its mechanism for neuroprotection. Nanomedicine. 2013.
Shanmugam S, Baskaran R, Balakrishnan P, Thapa P, Yong CS, Yoo BK.. Solid self-nanoemulsifying drug delivery system (S-SNEDDS) containing phosphatidylcholine for enhanced bioavailability of highly lipophilic bioactive carotenoid lutein. Eur J Pharm Biopharm. 2011; 79: 250-257.
Qi X, Wang L Zhu J, Hu Z, Zhang J., (2011). Self-double-emulsifying drug delivery system (SDEDDS): a new way for oral delivery of drugs with high solubility and low permeability. Int J Pharm. 409(1-2): 245-251.
Yin YM, Cui FD, Mu CF, Choi MK, Kim JS, Chung SJ, Shim CK, Kim DD. Docetaxel microemulsion for enhanced oral bioavailability: preparation and in vitro and in vivo evaluation. J Control Rel. 2009 Dec 3;140(2):86-94
Wang Y, Sun J, Zhang T, Liu H, He F, He Z. Enhanced oral bioavailability of tacrolimus in rats by self-microemulsifying drug delivery systems. Drug Dev Ind Pharm. 2011; 37: 1225-1230.
Mudra DR, Borchardt RT. Absorption barriers in the rat intestinal mucosa. 3: Effects of polyethoxylated solubilizing agents on drug permeation and metabolism. J Pharm Sci. 2010; 99: 1016-1027.
Li M, Si L, Pan H, Rabba AK, Yan F, Qiu J, Li G. Excipients enhance intestinal absorption of ganciclovir by P-gp inhibition: assessed in vitro by everted gut sac and in situ by improved intestinal perfusion. Int J Pharm. 2011; 403: 37-45.
Buyukozturk F, Benneyan JC, Carrier RL. Impact of emulsion-based drug delivery systems on intestinal permeability and drug release kinetics. J Control Rel. 2010; 142: 22-30.
Kogan A, Kesselman E, Danino D, Aserin A, Garti N. Viability and permeability across Caco-2 cells of CBZ solubilized in fully dilutable microemulsions. Colloids Surf B Biointerfaces. 2008; 66: 1-12.
Katneni K, Charman SA, Porter CJ. Impact of cremophor-EL and polysorbate-80 on digoxin permeability across rat jejunum: delineation of thermodynamic and transporter related events using the reciprocal permeability approach. J Pharm Sci. (2007); 96: 280-293.
Secretary, Govt of India, Ministry of Health ,Indian pharmacopoeia 2010, VIth Ed, New Delhi, India 2010
Tavano L, Muzzalupo R, Trombino S, Cassano R, Pingitore A, Picci N. Effect of formulations variables on the in vitro percutaneous permeation of Sodium Diclofenac from new vesicular systems obtained from Pluronic triblock copolymers. Colloids Surf B Biointerfaces. 2010; 79: 227-234.
Mehta SK, Kaur G, Bhasin KK. Analysis of Tween based microemulsion in the presence of TB drug rifampicin. Colloids Surf B Biointerfaces. 2007; 60: 95-104.
Kim SK, Lee EH, Vaishali B, Lee S, Lee YK, Kim CY, Moon HT, Byun Y. Tricaprylin microemulsion for oral delivery of low molecular weight heparin conjugates. J Control Rel. 2005; 105: 32-42.
Chaiyana W, Rades T, Okonogi S. Characterization and in vitro permeation study of microemulsions and liquid crystalline systems containing the anticholinesterase alkaloidal extract from Tabernaemontana divaricata. Int J Pharm. 2013; 452: 201-210.
Fernandez S, Jannin V, Chevrier S, Chavant Y, Demarne F, Carriere F. In Vitro Digestion of the Self-Emulsifying Lipid Excipient Labrasol by Gastrointestinal Lipases and Influence of its Colloidal Structure on Lipolysis Rate. Pharm Res. 2013;
Jiang L, Long X, Meng Q. Rhamnolipids enhance epithelial permeability in Caco-2 monolayers. Int J Pharm. 2013; 446: 130-135.
Larsen AT, Ohlsson, AG, Polentarutti B, Barker RA, Phillips AR, Abu-Rmaileh R, Dickinson PA, Abrahamsson B, Ostergaard J. et al. Oral bioavailability of cinnarizine in dogs: relation to SNEDDS droplet size, drug solubility and in vitro precipitation. Eur J Pharm Sci. 2013; 48: 339-350.
Wang W, Li CY, Wen XD, Li P, Qi LW. Simultaneous determination of 6-gingerol, 8-gingerol, 10-gingerol and 6-shogaol in rat plasma by liquid chromatography-mass spectrometry: Application to pharmacokinetics. J Chromatograpy B Analyt Technol Biomed Life Sci. 2009; 877: 671-679.
Kim MG, Shin BS, Choi Y, Ryu JK, Shin SW, Choo HW, Yoo SD Determination and pharmacokinetics of [6]-gingerol in mouse plasma by liquid chromatography-tandem mass spectrometry. Biomed Chromatogr. 2012; 26: 660-665.
Wahlang B, Kabra D, Pawar YB, Tikoo K, Bansal AK. Contribution of formulation and excipients towards enhanced permeation of curcumin. Arzneimittelforschung. 2012; 62: 88-93.