Formulation and Evaluation of Gastro Retentive Mucoadhesive Sustained Release Pellets of Acyclovir
Keywords:
Acyclovir (ACV), Pellets, Mucoadhesion strength (MS), iod. Keywords: Acyclovir (ACV), Pellets, Mucoadhesion strength (MS), Ex vivo residence (ES), DissolutionAbstract
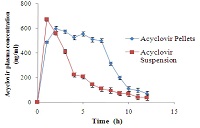
Acyclovir is an antiviral drug, belonging to the deoxyguanosine family, widely prescribed for the treatment of herpes simplex viral infections, as well as in the treatment of herpes zoster (shingles). Oral bioavailability of acyclovir is very low (10–20%) owing to its first pass metabolism with elimination half-life (t1/2) of 2-3 h. It has absorption window in upper gastrointestinal tract. Due to its rapid elimination from site of absorption and short biological half life, sustained release formulation system for acyclovir is advantageous. In this study, gastro retentive mucoadhesive SR pellets of acyclovir was prepared using HPMC K 100M as matrix former and Sodium CMC as mucoadhesive polymer by extrusion spheronization technique. Acyclovir pellets prepared with higher concentration of HPMC (batch G) showed in vitro drug release for 12 h with sufficient mucoadhesion strength and ex vivo resident time. Release kinetic studies indicated that drug release data had best fit to Higuchi’s model. In-vivo studies in rat model proved that relative bioavailability of acyclovir SR pellets get increased by 1.98 fold as compared plain drug suspension. The optimized formulation batch G was found to be stable during six months accelerated stability period.
References
. Clercq D, Erik F, Hugh J. Antiviral prodrugs, the development of successful prodrug strategies for antiviral chemotherapy, British Journal of Pharmacology, 2006; 147; P.1–11.
. O'Brien JJ, Campoli-Richards DM. Aciclovir: An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy, Drugs, 1989; 37; P.233–309.
. Sweetman S, editor. Martindale: The complete drug reference, 34th ed., London: Pharmaceutical Press, 2004; P. 550-554.
. Abrahamsson B, Alpsten M, Jonsson UE. Gastro-intestinal transit of a multiple unit formulation (metoprolol CR/ZOK) and a non-disintegrating tablet with the emphasis on colon, International Journal of Pharmacy, 1996; 140; P. 229-235.
. Dechesne JP, Delattre LA. New enteric tablet of acetylsalicylic acid:II Biopharmaceutical aspects. Intenational Journal of Pharmacy, 1986; 34; P. 259-262.
. Hogan J. Pharma-the science of dosage form design, New York: Churchill Livingstone, 2001; P. 441-448.
. Lyne CW, Johnston HG. The selection of pelletizers, Powder Technology, 1981; 29; P. 211-216.
. Wan LSC, Lai WF. Factors affecting drug release from drug-coated granules prepared by fluidized-bed coating. International Journal of Pharmacy, 1991; 72; P. 163-174.
. Eskilson C. Controlled release by microencapsulation. Manufacturing Chemist, 1985; 56; P. 33-41.
. Mitrevej A, Sinchaipanid N, Natpoolwat N, Naratikornrit N. Fabrication of multi unit controlled release phynylpropanamine hydrochloride tablets. Drug Development Industrial Pharmacy, 1998; 24; P. 793-796.
. Devices GSI. Pharmaceutical Pelletization Technology. New York: Marcel Dekker Inc. 1989; 37; P.30-100.
. Mali SL, Nighute AB, Deshmukh V. Microcrystals: for improvement of solubility and dissolution rate of lamotrigin , International Journal of Pharmaceutical Sciences, 2010; 2; P. 515-521.
. Ahmed TA, Mahmaud MF, Samy AM, Badawi AA, Gabr KE. Formulation, evaluation and optimization of miconazole nitrate tablet prepared by foam granulation technique, International Journal of Drug Delivery, 2011; 3; P. 712-733.
. Kagami Y, Sugimura S, Fujishima N, Matsuda K, Ometani T, Matsumura Y. Oxidative stability, structure, and physical characteristics of microcapsules formed by spray drying of fish oil with protein and dextrin wall materials, Journal of Food Sciences, 2003; 68; P. 2248-2255.
. Shanker G, Chegonda KK. Buccal drug delivery of tizanidine hydrochloride tablets, AAPS PharmSciTech, 2009; 10; P.530-539
. Singh SK, Bothara SB, Singh S, Patel R, Dodia R. Formulation and evaluation of mucoadhesive tablet: influence of some hydrophilic polymers on the release ate and in vitro evaluation, International Journal of Pharmaceutical Sciences and Nanotechnology, 2010; 3; P.1111-1121
. Vyas SP, Talwar N, Karajgi KS, NK Jain. An erythrocyte based bioadhesive system for nasal delivery of propranolol, Journal of Controlled Release, 1993; 23; P. 231–237.
. Harikarnpakdee S, Lipipun V, Sutanthavibul N, Ritthidej GC. Spray dried mucoadhesive pellets: preparation and transport through nasal cell monolayer. AAPS PharmSciTech, 2006; 7; P. 12.
. Harris MS, Tazeen J, Merchant HA, Yusuf RI. Evaluation of drug release kinetic from ibuprofen matrix tablets using HPMC. Pakistan Journal of Pharmaceutical Sciences, 2006; 19; P. 119-124.
. Palma-Aguirre JA, Absalon-Reyes JA, Novoa-Heckel G, Lago A, Oliva I, Rodriguez Z, la Parr Ma GD, Burke-Fraga V, Namur S. Bioavailability of two oral suspension and two oral tablet formulations of acyclovir 400mg: two single-dose, open-label, randomized, two-period crossover comparisons in healthy Mexican adult subjects, Clinical Therapeutics, 2007; 29: P. 1146–1152.
. International Conference on Harmonization, Q1A (R2): Stability Testing of New Drug Substances and Products, ICH, Geneva 2003.