Formulation Design and Optimization of Sustained Release Tablet of Ambroxol hydrochloride
Keywords:
Ambroxol hydrochloride, HPMC K15M, Eudragit RSPO, Direct compression, Factorial designAbstract
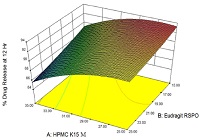
A sustained release matrix formulation for Ambroxol hydrochloride was designed and developed to achieve a 12 h release profile. Using HPMC K15M and Eudragit RSPO as an inert matrix forming agent to control the release of Ambroxol hydrochloride. The matrix tablets for these formulations were prepared by direct compression and their in-vitro release tests were carried out for a period of 12 hours using USP dissolution test apparatus (type I- Basket) at 37±0.5°C and 100 rpm speed. A 32 full factorial design was used for optimization by taking the concentration of HPMC K15M (X1) and Eudragit RSPO (X2) were selected as independent variables, where as initial release at the 2 hrs (Y1, % drug release), release rate at the 8 hrs (Y2, % drug release) and the concentration of Ambroxol hydrochloride released in 12 hrs (Y3, % drug release) were chosen as dependent variables. The optimized formulation F4 follows Hixon Croswell order release kinetics with nonFickian diffusion mechanism. From the study, it was concluded that the release of Ambroxol hydrochloride can be effectively sustained using combination of HPMC K15M and Eudragit RSPO.
References
Leon Lachmen, Herbert A Liberman, Joseph L kanig. Sustained release dosage forms, the theory and practice of industrial pharmacy, 3rd ed. Vargheese publishing house: Bombay; 1991. p. 430.
Venkatraman S, Davar A, Chester A, Kleiner L and Wise DL. Handbook of pharmaceutical controlled release technology: An overview of controlled release systems, New York, Marcel Dekker Inc, 2000. 431-465.
Brahmankar HA, Jaiswal SB. Biopharmaceutics and pharmacokinetics-A Treatise Vallabh Prakashan, New Delhi. 2000: 348-357.
Martindale. In, The Complete Drug Reference. 32th ed. The pharmaceutical press, London, 1999; 1054-1055.
Barar FSK Eds., In; Essentials of pharmacotherapeutics, 3rd ed. S. Chand and Company Ltd., New Delhi, 2005. p. 550.
Vergin H, Bishop Freudling GB, Miczka M, Nitsche V, Stroble K. Arzneim. Forsch-Drug Research 1985:35,1591.
Alighieri T, Avanessian S, Berlini S, Bianchi SG, Deluigi P, Valducci R, et al. Arzeim. Forsch Drug Research 1988:38,92.
Basak SC, Jayakumar RBM, Lucas MKP. Formulation and release behavior of sustained release ambroxol hydrochloride HPMC matrix tablet. Indian J Pharm Sci 2006:68:594-8.
Lee HJ, Joung SK, Kim YG, Yoo JY, Han SB. Bioequivalence assessment of ambroxol tablet after a single oral dose administration to healthy male volunteers. Pharm Research 2004;49:93-8.
Villacampa J, Alchntar F, Rodriguez JM, Morales JM, Herrera J, Rosete R. Pharmacokinetic properties of single-dose loratadine and ambroxol alone and combined in tablet formulations in healthy men. Clin Ther 2003; 25:2225-32.
British Pharmacopoeia. Cambridge: University Printing House; 2002; 211.
Sako K, Sawada T, Nakashima H, Yokohama S, Sonobe T. Influence of water soluble fillers in hydroxyl propyl methyl cellulose matrices on in-vitro and in-vivo drug release. J Control Release 2002; 81:165-172.
Williams III RO, Reynolds TD, Cabelka TD, Sykora MA, Mahaguna V. Investigation of excipient type and level on drug release from controlled release tablets containing HPMC. Pharm Dev Technol 2002; 7: 81-193.
Durig, T, Fassihi R. Guar-based monolithic matrix systems: Effect of ionizable and non-ionizable substances and excipients on gel dynamics and release kinetics. J Control Release 2002;80: 45–56.
Shahla Jamzad, Reza Fassihi. Development of a controlled release low dose class II drug-Glipizide. International J of Pharmaceutics 312,2006;24-32
Rowe RC, Sheskey PJ. Handbook of pharmaceutical excipients 6th edition. Pharmaceutical press; London: 2009;525-533.
Lee BJ, Ryu SG, Cui JH. Drug Dev Ind Pharm 1999;25:493-501
Rowe RC and Sheskey PJ. Handbook of Pharmaceutical Excipients 6th edition. Pharmaceutical press; London: 2009; 525-533.
Boza, A, Carabello I, Alvarez-Fuents J, Rabasco AM. 1999. Evaluation of Eudragit RSPO and Ethocel 100 matrices for the controlled release of lobenzarit disodium. Drug Dev Ind Pharm 25, 229-/233.
Mitrevej A, Sinchaopanid N, Natpoolwat N, Naratikornrit N. Fabrication of multiunit controlled release phenyl propanolamine hydrochloride tablets. Drug Dev Ind Pharm 1998;24: 793-796.
S Azarmia, J Farida A, Nokhodchib SM. Bahari-Saravia H. Thermal treating as a tool for sustained release of indomethacin from Eudragit RS and RL matrices International J of Pharmaceutics 2002;171-177
Joshi A, Pund S, Nivsarkar M, Vasu K, Shishoo C. Dissolution test for site specific release isoniazid pellets in USP apparatus 3 (reciprocating cylinder): Optimization using response surface methodology. European Journal of Pharmaceutics and Biopharmaceutics. 2008;69: 769–775.
Singh B, Dahiya M, Saharan V and Ahuja N. Optimizing drug delivery systems using systematic “design of experiments” Part II: Retrospect and prospects, Critical Reviews in Therapeutic Drug Carrier System. 2005;22:215-293.
Singh B, Mehta G, Kumar R, Bhatia A, Ahuja N and Katare OP. Design development and optimization of nimesulide loaded liposomal systems for topical application. Current Drug Delivery 2005;2:143-153.
Meka VS, Nali SR, Battu JR, Venkata RMK. Statistical design and evaluation of a propranolol HCl gastric floating tablet Acta Pharmaceutica Sinica B 2012;2(1): 60-69.
Lazarus J, Cooper J. Absorption, testing, and clinical evaluation of oral prolonged action drugs. J Pharm Sci 1961; 50:715–32.
Wagner JG. Interpretation of percent dissolved-time plots derived from in-vitro testing of conventional tablets and capsules. J Pharm Sci 1969; 58:1253–7.
Higuchi T. Mechanism of sustained action medication: theoretical analysis of rate release of solid drugs dispersed in solid matrices. J Pharm Sci 1963; 52:1145–9.
Korsemeyer R, Gurny R, Peppas N. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 1983; 15: 25–35.
Hixson AW, Crowell JH. Dependence of reaction velocity upon surface and agitation (I) theoretical consideration. Ind J Chem Eng 1931; 23:923–31.