Analytical Method Development and Validation for the Simultaneous estimation of Alprazolam and Propranolol in their combined dosage form
Keywords:
Alprazolam, Propranolol, Simultaneous Equation, Absorption Ratio, ValidationAbstract
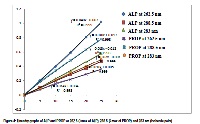
To develop and validate simple, rapid, economic and accurate spectrophotometric methods for the estimation of the Alprazolam and Propranolol which can be applied successfully for the analysis of both the drugs in pure as well as in their combined dosage form. A double-beam Shimadzu UV-Visible spectrophotometer, 1800, with a pair of 1cm matched quartz cells was used to measure the absorbance of the solutions in both developed methods viz. simultaneous equation method and absorption ratio method. 0.1N HCl was selected as solvent for the preparation of solutions. After scanning 10μg/ml solution of each drug separately in the range of 200-400 nm, wavelength of maximum absorption of ALP (262.5 nm) and PROP (288.5 nm) were selected for simultaneous equation method and isobestic point (283 nm) and absorption maxima of ALP (262.5 nm) were selected for absorption ratio method. The methods were validated statistically as per ICH guidelines. Linearity ranges from 5-25 μg/ml for both drugs. %RSD calculated was less than equal to 2 which indicates accuracy and reproducibility of the method. Recovery studies indicate that these drugs could be quantified simultaneously without interference of the excipients present in formulation. The developed UV spectroscopic methods are simple, precise, less time consuming, economical and accurate and thus are suitable for the analysis of ALP and PROP in combined dosage form.
References
Indian pharmacopoeia (1996) Govt. of India, Ministry of health and family welfare Delhi 1: 34-35.
Mandrioli R, Mercolini L, Raggi MA. Benzodiazepine metabolism: an analytical perspective. Current Drug Metabolism. 2008; 9 (8): 827–44.
Rani GT, Shankar DG, Kadgapathi P, Satyanarayana B. A Validated RP HPLC Method for Simultaneous Determination of Propranolol hydrochloride and Alprazolam in Bulk and in Pharmaceutical formulations. Journal of Pharmacy Research, 2011; 4(2): 358-360.
Shukla S, Kumar P, Narayana Moorthy NSH, Shrivastava SK, Trivedi P, Srivastava RS. RP-HPLC Method Development and Its Validation for Simultaneous Estimation of Alprazolam and Fluoxetine Hydrochloride in Pharmaceutical Dosage Form. Eurasian Journal of Analytical Chemistry 2010; 5(3): 239-245.
Young R, Glennon RA. S(-)Propranolol as a discriminative stimulus and its comparison to the stimulus effects of cocaine in rats. Psychopharmacology (Berl). 2009; 203 (2): 369–82.
Patil AS, Shirkhedkar AA, Surna SJ, Nawale PS. Q-Absorbance and Multicomponent UV Spectrophotometric Methods for Simultaneous Estimation of Propranolol Hydrochloride and Flunarizine Dihydrochloride in Capsules. Der Pharma Chemica, 2011; 3(3): 404-408.
Rani GT, Shankar DG, Kadgapathi P, Satyanarayana B. Development of an RP-HPLC Method for the Simultaneous Estimation of Propranolol Hydrochloride and Diazepam in Combined Dosage form. Indian Journal of Pharmaceutical Education and Research, 2011; 45(4): 296-300.
Kumar AK, Mohanakrishna A, Sudheer M, Rajesh KS and Ramalingam P. UV Spectrophotometric Method for the estimation of Alprazolam in Tablet Dosage Form, International Journal of ChemTech Research. 2011; 3(1): 161-164.
Patel RB, Patel AB, Patel MR, Shankar MB and Bhatt KK. Estimation of alprazolam and sertraline in pure powder and tablet formulations by high-performance liquid chromatography and high-performance thin-layer chromatography. Analytical Letters. 2009; 42(10-12): 1588-1602.
Patel RB, Patel MR, Shankar MB, Bhatt KK. Simultaneous determination of alprazolam and fluoxetine hydrochloride in tablet formulations by high-performance column liquid chromatography and high-performance thin-layer chromatography, Journal of AOAC International. 2009; 92(4): 1082-1088.
Lozano PP, Montoya EG, Orriols A, Minarro M, Tico JR, SuneNegre JM. Development and validation of a new HPLC analytical method for the determination of alprazolam in tablets. Journal of Pharmaceutical and Biomedical Analysis. 2004; 34: 979–987.
Gonsalves AR, Pineiro M, Martins JM, Barata PA, Menezes JC. Identification of Alprazolam and its degradation products using LC-MS-MS, ARKIVOC. 2010; 5: 128-141.
Hanysova L, Grafetterova T, Dubovska M, Klimes J. Development of the analytical method for LC-MS detection of unknown degradation product of Alprazolam, Chemical Papers. 2005; 59 (2): 99-102.
Nudelman NS, Cabrera CG, Spectrofluorimetric assay for the photodegradation products of Alprazolam, Journal of Pharmaceutical and Biomedical Analysis. 2002; 30(3): 887-893.
Shingbal DM, Prabhudesai JS. Spectrophotometric estimation of propranolol hydrochloride. Indian Drugs. 1984; 21(7): 304-305.
El-Didamony AM. Sensitive spectrophotometric method for the determination of propranolol HCl based on oxidation bromination reactions, Drug testing and analysis. 2010; 2(3): 122-129.
Modamio P, Lastra CF, Marino EL. Error structure for the HPLC analysis for atenolol, metoprolol and propranolol: a useful weighting method in parameter estimation. Journal of Pharmaceutical and Biomedical Analysis. 1998; 17(3): 507-13.
Salman SA, Sulaiman SA, Ismail Z, Gan SH. Quantitative determination of propranolol by ultraviolet HPLC in human plasma. Toxicology Mechanisms & Methods. 2010; 20(3): 137-42.
Venkatesh G, Ramanathan S, Mansor SM, Nair NK, Sattar MA, Croft SL, Navaratnam V Development and validation of RP-HPLC-UV method for simultaneous determination of buparvaquone, atenolol, propranolol, quinidine and verapamil: a tool for the standardization of rat in situ intestinal permeability studies, Journal of Pharmaceutical and Biomedical Analysis. 2006; 43(4): 1546-1551.
Souri E, Farsam H, Amini L. Stereospecific determination of propranolol by high performance liquid chromatography using UV detection. Daru. 1999; 7(2): 18-21.
Bhavar G, Chatpalliwar VA. Quantitative analysis of propranolol hydrochloride by high performance thin layer chromatography. Indian Journal of Pharmaceutical Sciences, 2008; 70(3): 395-398.
ICH Topic Q 2 (R1) Validation of Analytical Procedures: Text and Methodology, note for guidance on validation of analytical procedures: text and methodology (CPMP/ICH/381/95), June 1995.
International Conference on Harmonization; Draft Guidance on specifications: Test Procedures and Acceptance Criteria for New Drug Substances and Products: Chemical Substances, Federal Register (Notices), 65 (251), 2000, 83041–83063.