Development and characterization of solid lipid dispersion as delivery system for hydrophilic antihypertensive drug atenolol
Keywords:
Atenolol, water soluble drug, lipophilic excipients, solid dispersion, sustained release, permeationAbstract
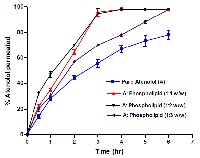
Atenolol is a hydrophilic β-blocker drug characterized by high solubility and low permeability which corresponds to BCS class III drug. The purpose of the study was to develop solid dispersion of atenolol with fatty excipients to modify the release and enhance intestinal permeability of the drug. The solid dispersions containing atenolol were prepared using lipophilic surfactants, saturated fatty acid, triglycerides and phospholipids by co-evaporation method. The obtained solid dispersions were characterized by differential scanning calorimetry, infrared spectroscopy, drug solubility, % yield, % encapsulation efficiency and in vitro drug release. The results of in vitro release studies indicated that drug release from the drug: phosphotidylcholine dispersion (1:1w/w) showed a sustained release in comparison with the pure atenolol and the other solid dispersions. The influence of phosphotidylcholine on drug intestinal permeation was further evaluated versus pure drug. The results of in vitro permeability revealed that drug-phosphotidylcholine solid dispersion significantly enhanced % permeation of atenolol in comparison with the pure drug. This could be attributed to higher lipophilicity acquired by incorporation of the drug within the solid lipid dispersion. On the basis of the result obtained, it was concluded that solid dispersion of atenolol with phosphotidylcholine is a good approach to modify the release and enhance permeability of water soluble drug. However, the influence of lipophilic solid dispersion on atenolol bioavailability needs further investigation..
References
Koga K, Takarada N, Takada K. Nano-sized water-in-oil-in-water emulsion enhances intestinal absorption of calcein, a high solubility and low permeability Compound. Eur. J .Pharm. Biopharm. 2010, 74 (2), 223-32.
Wang S, Sun M, Ping Q. Enhancing effect of labrafac lipophile WL 1349 on oral bioavailability of hydroxysafflor yellow A in rats. Int. J. Pharm. 2008 Jun 24,358(1-2), 198-204.
Takada K. Oral delivery of hematopoietic factors: progress with gastrointestinal mucoadhesive patches, microdevices, and other microfabrication technologies. Am. J. Drug Del. 2006, 4, 65–77.
Deshmukh DD, Ravis WR, Betageri GV. Improved delivery of cromolyn from oral proliposomal beads. Int .J. Pharm. 2008 24,358(1-2), 128-164.
Fricker G, Kromp T, Wendel A, Blume A, Zirkel J, Rebmann H, Setzer C, Quinkert RO, Martin F, Müller-Goymann C. Phospholipids and lipid-based formulations in oral drug delivery. Pharm. Res. 2010, 27(8), 1469-1555.
Ali O, Obaid R, Saify ZS, Ahmed SW. Biopharmaceutical evaluation of oral tablet Atenolol (100 and 50 mg) on local population. Pak. J. Pharm. Sci. 2005, 18(1), 25-32.
Milind P, Wagh J, Patel S. Biopharmaceutical Classification System: Scientific Basis for Bilayer Extensions Int. J.Pharm. Pharm.Sc. 2010, 2(1), 12-19.
Jug M, Bećirević-Laćan M, Bengez S. Novel cyclodextrin-based film formulation intended for buccal delivery of atenolol. Drug. Dev. Ind. Pharm. 2009 35(7), 796-807.
Wu F, Cheni P, Lee YJ, Long RR. Comparative Pharmacokinetics of Two Atenolol Products .Journal of Food and Drug Analysis.2003, 4-7.
Sastry SV, Reddy IK, Khan MA. Atenolol gastrointestinal therapeutic system: Optimization of formulation variables using response surface methodology. J. Control. Release. 1997, 45,121-130.
Singh B, Kaur S, Chakkal AN. Formulation and Optimization of Controlled Release Mucoadhesive Tablets of Atenolol Using Response Surface Methodology .AAPS. Pharm.Sci.Tech. 2006, 7(1), 3.
Vaithiyalingam SR, Sastry SV, Dehon RH, Reddy IK, Khan MA. Long- term stability characterization of a controlled release gastrointestinal therapeutic system coated with cellulose acetate pseudolatex. Pharmazie. 2001, 56, 66-69.
Kim J, Shin SC. Controlled release of atenolol from the ethylene-vinyl acetate matrix. Int. J. Pharm. 2004,273, 23-27.
Jug M, Bećirević-Laćan M, Bengez S. Novel cyclodextrin-based film formulation intended for buccal delivery of atenolol. Drug Dev. Ind. Pharm. 2009, 35(7), 796-807.
Kulkarni A, Bhatia M. Development and Evaluation of Regioselective Bilayer Floating Tablets of Atenolol and Lovastatin for Biphasic Release Profile. Iranian J. Pharma. Res. 2009, 8 (1), 15-25.
Srivastava AK, Wadhwa S, Ridhurkar D, Mishra B. Oral Sustained Delivery of Atenolol from floating Matrix Tablets—Formulation and in vitro Evaluation. Drug .Dev. Ind. Pharm. 2005, 31(4-5), 367-74.
Nokhodchi A, Talari R, Valizadeh H, Jalali MB. An investigation on the solid dispersions of chlordiazepoxide. Int. J. Bio. 2007, 3,211- 217.
Hammady T, El-Gindy A, Lejmi E, Dhanikula RS, Moreau P, Hildgen P. Characteristics and properties of nanospheres co-loaded with lipophilic and hydrophilic drug models. Int. J. Pharm. 2009, 18,369(1-2), 185-95.
Smith PL. Methods for evaluating intestinal permeability and metabolism in-vitro. Pharm. Biotechnol. 1996, 8, 13-34.
Rafael NP, Bruno RV, Ariane PC, Talize F, Fabio SM, Marcos AS. Thermo analytical Study of Atenolol and Commercial Tablets. Lat. Am. J. Pharm. 2007, 26, (3), 382-388.
Sharma P, Varma MV, Chawla HP, Panchagnula R. Relationship between lipophilicity of BCS class III and IV drugs and the functional activity of peroral absorption enhancers. Farmaco. 2005, 60(11-12), 870-873.
Wang T, Wang N, Hao A, He X, Li T, Deng Y. Lyophilization of water- in-oil emulsions to prepare phospholipid-based anhydrous reverse micelles for oral peptide delivery. Eur .J .Pharm. Sci. 2010, 18, 39(5), 373-382.
Kawaguchi E, Shimokawa K, Ishii F. Physicochemical properties of structured phosphotidylcholine in drug carrier lipid emulsions for drug delivery systems. Colloids Surf. B. Biointerfaces. 2008, 62(1), 130-135.
Robhash KS, Keon WK, Hoo-Kyun C. Preparation and characterization of solid lipid nanoparticles loaded with doxorubicin. Eur J .Pharm. Sci. 2009, 37, 508–513.