Rapid and selective UV Spectrophotometric method for the analysis of Olmesartan medoxomil in bulk and dosage form.
Keywords:
Olmesartan medoxomil, UV-Spectrophotometry, RecoveryAbstract
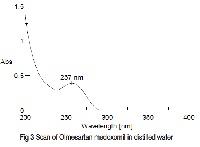
A new UV spectrophotometric method was developed for quantitative evalution of Olmesartan medoxomil preparations. The UV detector was set at 256nm. Beers law is obeyed in the concentration range of 2.0 -14.0 øg/ml. The method was found to be selective, linear, accurate and precise in the specified ranges. Intra and interday variability for the method were < 2% relative standard deviation common excipients used as additives in pharmaceutical preparations do not interfere with the proposed method. This method was successfully used for quantification of Olmesartan medoxomil in pure form and in pharmaceutical preparations.
References
Mire DE, Silfani TN, Pugsley MK. A Review of the structural and functional features of Olmesartan medoxomil, an angiotensin receptor. J Cardiovasc Pharm 2005; 46: 585–593.
Kobayashi N, Fujimori I, Watanabe M, Ikeda T. Real – time monitoring of metabolic reactions by microdialysis in combination with tandem mass spectrometry; hydrolysis of CS-866 in vitro in human and rat plasma, livers and small intestines. Anal Biochem 2000; 287: 272– 278.
Yanagisawa H, Amemiya Y, Kanazaki T,Shimoji Y, Fujimoto K, Kitahara Y, Sada T, Mizuno M, Ikeda M, Miyamoto S, Furukawa Y, Koike H.Nonpeptide angiotensin II receptor antagonists; synthesis, biological activities and structure- activity relationships of imidazole -5 carboxylic acids bearing alkyl,alkenyl and hydroxyalkyl substituents at the 4-position and their related compounds. J Med Chem 1996; 39: 323–338
Unger T, McInnes GT, Neutel JM, Bo¨hm M. The role of Olmesartan medoxomil in the management of hypertension. Drugs 2004; 64: 2731–2739.
Bari P D, Rote A R. RP-LC and HPTLC methods for the determination of olmesartan
medoxomil and hydrochlorothiazide in combined tablet dosage forms, Chromatographia,
; 69: 1469-1472.
Bajerski L, Rossi R C, Dias C L, Bergold A M, Froehlich P E. Stability indicating LC determination of a new antihypertensive, Olmesartan medoxomil in tablets. Chromatographia 2008; 68: 991-996.
Sagirli O, Onal A, Toker SE, Sensory D. Simultaneous HPLC analysis of olmesartan
and hydrochloride in combined tablets and in vitro dissolution studies. Chromatographia 2007; 66:213.
Murakami T, Konna H, Fukulsu N,Onodera M , Kawasaki T, Kusu F. Identification of
a degradation product in stressed tablets of olmesartan medoxomil by the complementary
use of HPLC hyphenated techniques. J Pharm Biomed Anal 2008;47: 553-559.
Lui D, Hu P, Matsushima N, Li X, Li L, Jiang J. Quantitative determination of olmesartan in human plasma and urine by liquid chromatography coupled to tendem mass spectrometry. J Chromatogr B.Analyt Technol Biomed Life Sci 2007; 856:190-197.
Nahamura H, Inoue T, Arakawa N, Shimuzi Y, Yoshigae Y, Fujimori I et al,. Pharmacological and pharmacokinetic study of Olmesartan medoxomil in diabetic retinopathy models. Eur J Pharmocol 2005; 512: 239-246.
Vaidya V V, Roy S M N, Yetal S M, Joshi S S, Parekh S A. LC-MS-MS Determination of Olmesartan in human plasma. Chromatographia. 2008; 67:147-150.
Celebier M, Altinoz S. Development of a Capillary Zone Electrophoresis Method for the Indirect Determination of Olmesartan Medoxomil and Determination of
Hydrochlorothiazide in Synthetic Tablets. Hacettepe University Journal of the Faculty of Pharmacy 2007; 27 (2): 119-130.
Altinoz S, Celebier M. Determination of Olmesartan medoxomil in tablets by UV-Vis
spectrophotometry. Pharmazie 2007; 62:419-422.