n vivo evaluation of newly developed losartan potassium sustained release dosage form using healthy male Indian volunteers.
Keywords:
In vivo evaluation, Sustained release, Dissolution, pharmacokinetic studiesAbstract
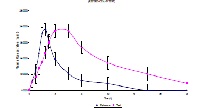
Sustained release (SR) matrix tablets of losartan potassium were prepared by wet granulation using xanthene as the polymer. The studies indicated that the drug release can be modulated by varying the concentration of the polymer and the fillers. The estimation of losartan potassium from human plasma method involves simple protein precipitation techniques using nifedipine as internal standard. Chromatographic separation was carried out on a reversed phase C18 column using mixture of 0.5% triethyl amine (pH 3.5) and acetonitrile (60:40, v/v) at a flow rate of 1.0 mL/min with UV detection at 225 nm. The method was validated and found to be linear in the range of 20-300 ng/ml. An open, randomized, two-treatment, two period, single dose crossover, bioavailability study in 24 fasting, healthy, male, volunteers was conducted. Various pharmacokinetic parameters including AUC0ăt, AUC0ă¥, Cmax, Tmax, T1/2, and elimination rate constant (Kel) were determined from plasma concentration of both formulations. These results indicated that the analytical method was linear, precise and accurate. The sustained and efficient drug delivery system developed in the present study will maintain plasma losartan potassium levels better, which will overcome the drawbacks associated with the conventional therapy
References
Brenner BM, Cooper ME, de Zeeuw D. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. Engl J Med 2001; 345: 861–9.
Deanne L, Hertzog, Jennifer Finnegan McCafferty, Xueguang Fang, Jeffrey Tyrrell R, Robert A, Reed. Development and validation of a stability-indicating HPLC method for the simultaneous determination of Losartan potassium, hydrochlorothiazide, and their degradation products. J Pharm Biomed Anal 2002; 30: 747-760.
Rudy Bonfilio, Cesar Ricardo Teixeira Tarley, Gislaine Ribeiro Pereira, Herida Regina Nunes Salgado Magali Benjamim de Araujo. Multivariate optimization and validation of an analytical methodology by RP-HPLC for the determination of losartan potassium in capsules. Talanta 2009;80: 236-41.
Nevin Erk. Analysis of binary mixtures of losartan potassium and hydrochlorothiazide by using high performance liquid chromatography, ratio derivative spectrophotometric and compensation technique. J Pharm Biomed Anal 2001;24:603-611.
Zhongxi (Zack) Zhao, Qingxi Wang, Eric W Tsai, Xue-Zhi Qin, Dominic Ip.Identification of losartan degradates in stressed tablets by LC-MS and LC-MS/MS. J Pharm Biomed Anal 1999; 20: 129-36.
Kathleen E, McCarthy, Qingxi Wang, Eric W Tsai, Rebecca E, Gilbert,Dominic P.Ip, Marvin A. Brooks. Determination of losartan and its degradates in COZAAR® tablets by reversed-phase high-performance thin-layer chromatography. J PharmBiomed Anal1 998; 17: 671-77.
Don Farthing., Domenic Sica., Itaf Fakhry., Antonio Pedro. And Todd W. B. Gehr, Simple high-performance liquid chromatographic method for determination of losartan and E-3174 metabolite in human plasma, urine and dialysate. J Chrom B biomed Sci Appln 1997; 19: 374-78.
Jing-Ying Jia, Meng-Qi Zhang, Yan-Mei Liu, Yun Liu, Gang-Yi Liu, Shui-Jun Li, Chuan Lu, Li-ping Weng, Yu-Lin Qi, Chen Yu. Pharmacokinetics and bioequivalence evaluation of two losartan potassium 50-mg tablets: A single-dose, randomized-sequence,open-label, two-way crossover study in healthy Chinese male volunteers. Clin Ther 2010; 32: 1387-95.
Michael A. Ritter, Christine I, Furtek, Man-Wai Lo, An improved method for the simultaneous determination of losartan and its major metabolite, EXP3174, in human plasma and urine by high-performance liquid chromatography with fluorescence detection. J Pharm Biomed Anal 1997; 15: 1021-29.
Williams, RC, Alasandro, MS, Fasone, VL, Boucher R J, Edwards J F. Comparison of liquid chromatography, capillary electrophoresis and super-critical Fluid chromatography in the determination of Losartan potassium drug substance in Cozaar®tablets. J Pharm Biomed Anal 1996; 14: 1539-46.
Budi Prasaja, Lucy Sasongko, Yahdiana Harahap, Hardiyanti, Windy Lusthom. And Matthew Grig, Simultaneous quantification of losartan and active metabolite in human plasma by liquid chromatography–tandem mass spectrometry using irbesartan as internal standard. J Pharm Biomed Anal 2009; 49: 862-867.
Maja Lusina, Tanja Cindric, Jadranka Tomaic, Marijana Peko, Lidija Pozaic, Nenad Musulin. Stability study of losartan/hydrochlorothiazide tablets. Int J Pharmace 2005; 291: 127-137.
Brain WW, Derek Cooper. Samples and standards, Analytical Chemistry by Open Learning, John Wiley and Sons, London. 1991; 2- 5.
Robert D.Braun., Introduction to Instrumental Analysis, 1stedn, McGraw Hill Book Company, 1987; 1-13.
Jeffery, GH, Basselt J, Vogel's Text Book of Quantitative Chemical Analysis, 1991; 5thedn. 217-35.
Adelbert, Knevel,M., Frank, E., Digangi, Jenkin's., Quantitative Pharmaceutical Chemistry. 1989; 21-23.
Sandy Lindsay, HPLC by open Learning, John Wiley and Sons, 30-45.
Lloyd R.Snyder, Joseph J.Kirkland. Joseph L.Glajch. Practical HPLC Method development, 1991.
Peppas, NA, Analysis of Fickian and nonfickian drug release from polymers. Pharm Acta Hel 1985; 60 110-111.
Higuchi T. Mechanism of sustained action medication: theoretical analysis of rate of release of solid drugs dispersed in sold matrices. J Pharm Sci 51963; 2: 1145-149.
Hosny EA, Al-Helw ARM, Al- ardiri MA. Comparative study of in-vitro release diclofenac sodium from certain hydrophilic polymers and commercial tablets in beagle dogs. Pharm Acta Helv 1997; 73: 159–164.
FDA Guidance for Industry, Bioanalytical Method Validation, US Department of Health and Human Services, Food and Drug Administration, 2001; Centre for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM).