In vitro evaluation of topical gel prepared using natural polymer
Keywords:
Topical gel, Bioadhesion, Natural polymerAbstract
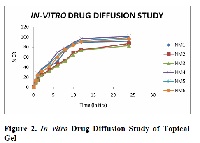
Nimesulide is a second generation non–steroidal anti–inflammatory agent, which is widely used in the long term therapy of rheumatoid arthritis, in alleviating pain and inflammation. But its short half-life (only 3–4 hr), so its causes more fluctuation. After oral administration Nimesulide causes to produces heart burn, nausea, loose motions, pruritus, etc. The present study based on the preparation of bioadhesive topical gel of Nimesulide, so as to avoid all gastric side effects. For the preparation of bioadhesive topical gel natural polymer aegel marmelos (plant Bale) was used. Bioadhesive polymers are the agents which increases the contact between the formulation and biological membrane, so as to avoid the fluctuation of formulation and behave as a sustained release formulation. In the present study, prepared bioadhesive topical gel was evaluated with the help of different parameters like drug content, spreadability, extrudability, swelling index study, in–vitro drug diffusion study, in-vitro drug release kinetic study and ex–vivo bioadhesive measurement. On the basis of in–vitro drug diffusion study and ex–vivo bioadhesive measurement property of gel, we have concluded that natural polymer aegel marmelos is the best polymer for the preparation of sustained release bioadhesive topical gel.
References
Sudhakar Y, Kuotsu K, Bandyopadhyay AK,
Buccal bioadhesive drug delivery – a promising
option for orally less efficient drugs, J. Control
Rel., 2006; 114: 15 – 40.
Jawahar N, Jayaprakash S, Maria Gerald Rajan NS,
Nagarajan M, Dhachina Moorthi D, Jubie S,
Manivannan R, Design and evaluation of sustained
release suppositories of Nimesulide, Indian J.
Pharm. Sci., 2005; 67(5): 558 – 561.
Derle DV, Sagar BSH, Pimpale R, Microemulsion
as a vehicle for transdermal permeation of
Nimesulide, Indian J. Pharma Sci., 2006; 68(5):
– 625.
Honrao MS, Pabari R, Gels, The Indian Pharmacist,
: 16 – 21.
Temu MJ, Damian F, Kinget R, Mooter GVD,
Intra-vaginal gels as drug delivery systems, J.
Women. Health, 2004; 13: 834 – 844.
Korsmeyer RW, Gurney R, Doelker E, Buri P,
Peppas NA, Mechanism of Solute Release from
Porous hydrophilic polymer, J. Pharma. Sci., 1983;
: 25-30.
Schmolka IR, Preparation and properties of
Pluronic PF -127 gels for the treatment of bruns, J.
Biomed. Mater. Res., 1972; 6: 571 – 582.
Chaudhari P, Ajab A, Malpure P, Kolsure P, Sanap
D, Development and in-vitro evaluation of thermo
reversible nasal gel formulations of Rizatriptan
benzoate, Indian J. Pharm. Edu. Res., 2009; 43: 55-
Saleem MA, Sanaullah S, Faizan S, Formulation
and Evaluation of Gatifloxacin topical Gel, The
Indian Pharmacist, 2006: 88-92.
Gupta GD, Gaud RS, Release rate of Nimesulide
from different gellants, Indian J. Pharm. Sci., 1999;
: 227 – 230.
Sanjay, Jain BD, Padsalg A, Patel K, Mokale V,
Formulation, development and evaluation of
Fluconazole gel in various polymer bases, Asi. J.
Pharm., 2007; 1: 63 – 68.
Gupta GD, Gaud RS, Release rate of Tenoxicam
from acrypol gels, The Indian Pharmacist., 2005:
– 76.
Mutimer MN, Riffkin C, Hill JA, Glickman ME,
Cyr GN, Modern ointment bases technology – II –
Comparative evaluation of bases, J. Am. Pharm.
Assoc., 1956; XLV: 212 – 218.
Chakole CM, Shende MA, Khadatkar SN,
Formulation and development of novel combined
halobetasol propionate and fusidic acid ointment,
Int. J. Chem. Tech. Res., 2009; 1: 103 – 116.
Rathore RPS, Nema RK, Formulation and
evaluation of topical gels of Ketoprofen, Asian. J.
Pharm. Clinical. Res., 2008; 1: 12 – 16.
Kamel AE, Sokar M, Naggar V, Gamal SA,
Chitosan and sodium alginate-based bioadhesive
vaginal tablets, AAPS PharmSci., 2002; 4: 1 – 7.
Mishra DN, Gilhotra RM, Design and
characterization of bioadhesive in – situ gelling
ocular insert of gatifloxacin sesquihydrate, Daru;
, 16: 1 – 8.
Patel RP, Patel G, Baria A, Formulation and
evaluation of transdermal patch of aceclofenac, Int.
J. Drug Del., 2009; 1: 41 – 51.
Gupta A, Garg S, Khar RK, Measurement of
bioadhesive strength of mucoadhesive buccal
tablets: design of an in-vitro assembly, Ind. Drugs,
; 30: 152 – 155.
Patel VM, Prajapati BG, Patel HV, Patel K,
Mucoadhesive bilayer tablets for Propranolol Hcl,
AAPS PharmSciTech., 2007; 8: E1 – E6.
Emami J, Varshosaz J, Saljoughian N,
Development and evaluation of controlled – release
buccoadhesive verapamil hydrochloride tablets,
Daru, 2008; 16: 60 – 69.